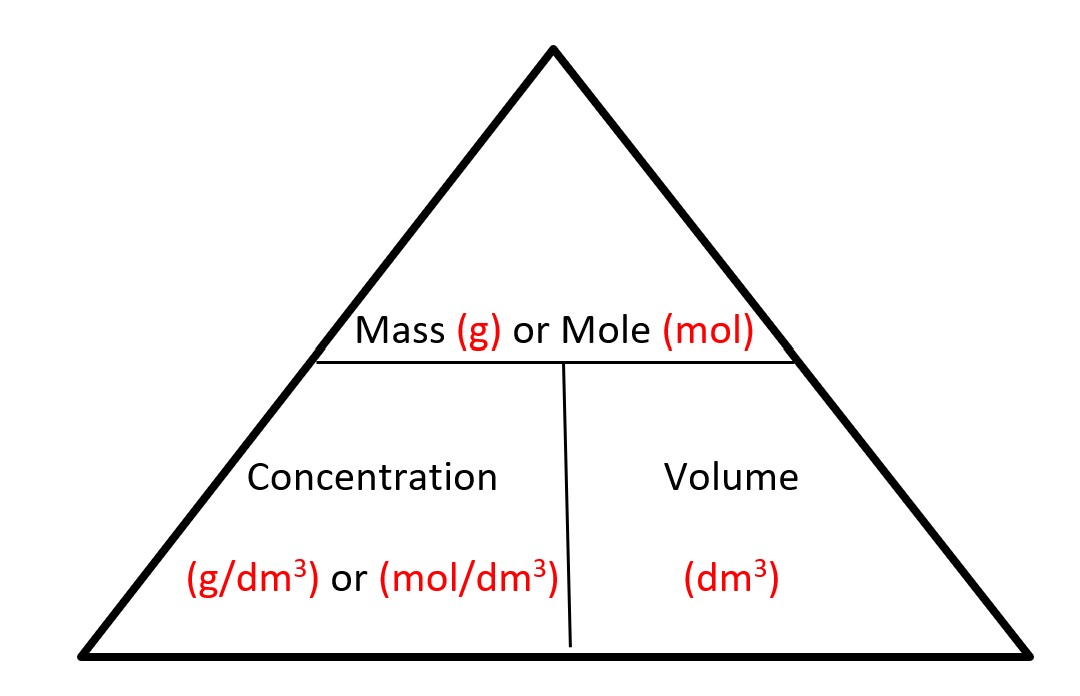
The See-Saw
| Mass | Volume | Number of Particles | |||
| mole | Ar/ Mr | Ξ | 24 dm3 at r.t.p | Ξ | 6.0 X 1023 |
Cations
| Coloured | White | None | |||||||||
| NaOH (aq) | NH3 (aq) | NaOH (aq) | NH3(aq) | NaOH (aq) | NH3(aq) | ||||||
| Cu2+ | I | *S | Zn2+ | S | S | Ca2+ | *I | - | |||
| Fe2+ | I | I | Al3+ | S | I | NH4+ | - | - | |||
| Fe3+ | I | I | Pb2+ | S | I | ||||||
Anions
| Coloured | White | None | |||||||||
| Reagent | Pdt | Reagent | Pdt | Reagent | Pdt | ||||||
| I- | H+ / Pb(NO3)2 | PbI2 | Cl- | H+ / AgNO3 | AgCl | NO3- |
Al/ NaOH Heat |
NH3 | |||
| SO42- | H+ / Ba(NO3)2 |
BaSO4
|
CO32- | H+ | CO2 | ||||||
| I | Pee | on | Ch SO |
Ag Ba |
with |
NO CO |
NH3 CO |
||||
(A) Naming of ionic compounds [ 'Metal' + 'Non-metal' ]
Pre-requisites
A1) Write on top of the Roman numerals of the Periodic Table 1+ 2+ 3+ ..... as shown below.
| 1+ | 2+ | 3+ | 4+/4- | 3- | 2- | 1- | 0 | |||
| I | II | III | IV | V | VI | VII | 0 |
A2) Highlight the elements as shown below. Call this 'a 7 and a dot'. These standalone elements will always be
written as X2. They are diatomic.
| I | II | III | IV | V | VI | VII | 0 | |||
| H | ||||||||||
| N | O | F | ||||||||
| Cl | ||||||||||
| Br | ||||||||||
| I | ||||||||||
| At |
A3) Memorise these commonly used polyatomic ions.
| NH4+ | NO3- | OH- | HCO3- | CO32- | SO42- | SO32- | PO43- | ||
| ammonium | nitrate | hydroxide | hydrogencarbonate | carbonate | sulfate | sulfite | phosphate |
A4) For metal ions with Roman Numerals, they are either transition metals or Group IV metals, the charge of the
metal ion follows the Roman Numerals assigned.
(i) iron (II) ... means charge of iron is 2+. It is written as Fe2+.
(ii) iron (III) ... means charge of iron is 3+. It is written as Fe3+.
(iii) copper (I) ... means charge of copper is 1+. It is written as Cu+.
(iv) copper (II) ... means charge of copper is 2+. It is written as Cu2+.
(v) lead (II) .... means charge of lead is 2+. It is written as Pb2+.
(vi) lead (IV) .... means charge of lead is 4+. It is written as Pb4+.
Q1 : Chemical formula of calcium chloride.
A1 : Step 1 : Now, look at the Periodic Table and refer to A1. Write down Ca2+ and Cl-.
Step 2 : Cross 'number only NOT CHARGES' to bottom of the element. Hence, Ca is 1 and Cl is 2 like this. Ca1Cl2
Step 3 : There is no need to put 1 for Ca as it is understood as '1' NOT '0'. Therefore, final answer : CaCl2
Q2 : Chemical formula of sodium chloride.
A2 : Step 1 : Now, look at the Periodic Table and refer to A1. Write down Na+ and Cl-.
Step 2 : If the numerically charges are the same (e.g 1+ 1- or 2+ 2- or 3+ 3- , ignore crossing and simply write as, in
this case, NaCl.)
Q3 : Chemical formula of calcium hydroxide.
A3 : Step 1 : Now, look at the Periodic Table and refer to A1, write down Ca2+. Refer to A3, write down OH-.
Step 2 : Cross 'number only NOT CHARGES' to bottom of the ions. Hence, Ca is 1 and OH is 2 like this Ca1OH2
Step 3 : Since OH- is polyatomic ion, bracket (OH). Ignore '1' for Ca to get the final answer Ca(OH)2.
Q4 : Chemical formula of iron (III) sulfate.
A4 : Step 1 : Refer to A4, write down Fe3+. Refer to A3, write down SO42-.
Step 2 : Cross 'number only NOT CHARGES' to bottom of the ions. Hence, Fe is 2 and SO4 is 3 like this Fe2SO4 3
Step 3 : Since SO42- is polyatomic ion, bracket (SO4) to get the final answer Fe2(SO4)3.
(B) Naming of covalent compounds [ Non-metal + Non-metal ]
Pre-requisites
B1) Memorise the prefixes of the following.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| mono- | di- or bi- | tri- | tetra- | penta- | hexa- | hepta- | octa | nona- | deca- |
Q1 : Chemical formula of carbon dioxide.
A1 : Step 1 : Now, look at the Periodic Table and look for the symbol of carbon. Write down C.
Step 2 : Oxide comes from the element oxygen. Now look for symbol oxygen. Write down O.
Step 3 : Refer to B1. 'di' means 2. Therefore, final answer is CO2. Note : O comes first, then 2.
Q2 : Chemical formula of sulfur trioxide.
A2 : Step 1 : Now, look at the Periodic Table and look for the symbol of sulfur. Write down S.
Step 2 : Oxide comes from the element oxygen. Now look for symbol oxygen. Write down O.
Step 3 : Refer to B1. 'tri' means 3. Therefore, final answer is SO3. Note : O comes first, then 3.
Q3 : Chemical formula of dinitrogen tetroxide.
A4 : Step 1 : Now, look at the Periodic Table and look for the symbol of nitrogen. Write down N.
Step 2 : Oxide comes from the element oxygen. Now look for symbol oxygen. Write down O.
Step 3 : Refer to B1. 'di' means 2 and 'tetra' means 4. Therefore, final answer is N2O4.
Note : N comes first, then 2. O comes first, then 4.
Suppose you follow the writing of the chemical formula, the name should be sulfur oxide-tri or carbon oxide-di. As this naming
sounds awkward, the naming convention follows that the prefix precedes the element.
(C) Naming of acids and bases
Pre-requisites
C1) Mineral acids have a 'H' in front while inorganic bases have an 'O' or 'OH' behind.
C2) Aqueous ammonia is the only base without 'O' or 'OH' behind in the chemical formula and it is written as
NH3 (aq).
(Note : Ammonium hydroxide does not exist.)
C3) Organic acids have a '-COOH' behind while organic bases have a '-NH2' behind.
C4) Prefixes of organic compounds.
| No. of carbon atoms | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Prefix | meth- | eth- | prop- | but- | pent- | hex- | hept- | oct- | |
| Structural Prefix | CH3 | C2H5 | C3H7 | C4H9 | C5H11 | C6H13 | C7H15 | C8H17 |
Q1 : Chemical formula of sulfuric acid.
A1 : Step 1 : Refer to C1, write down H+. Refer to A3, write down SO42- as sulfuric comes from sulfate.
Note : All '-ric' comes from '-ate' & '-rous' comes from '-ite'.
Step 2 : Cross 'number only NOT CHARGES' to bottom of the ions. Hence, H is 2 and SO4 is 1 like this H2SO4 1
Step 3 : SO42- is polyatomic ion, bracket (SO4)1 but this is redundant as (SO4)1 is understood as SO4.
Final answer : H2SO4.
Q2 : Chemical formula of ethanoic acid.
A2 : Step 1 : Refer to C3, write down COOH as it is an organic acid, -oic acid. Refer to C4, write CH3 NOT C2H5 as the
C in COOH contributes to 1 carbon. Naming is based on the number of carbon atoms present in compound.
Step 2 : Combine them and you get CH3COOH.
Q3: Chemical formula of iron (III) hydroxide.
A4 : Step 1 : Refer to A4, write down Fe3+. Refer to A3, write down OH-.
Step 2 : Cross 'number only NOT CHARGES' to bottom of the ions. Hence, Fe is 1 and OH is 3 like this Fe1 OH 3
Step 3 : Since OH- is polyatomic ion, bracket (OH) and ignore '1' to get the final answer Fe(OH)3.
Q4 : Chemical formula of methylamine.
A4 : Step 1 : Refer to C3, write down NH2 as it is an organic base, -amine. Refer to C4, write CH3 as it is a methyl.
Step 2 : Combine them and you get CH3NH2.
General rule of the thumb : Non-metal + Non-metal --> Covalent
STEP 1
Identify the Group the non-metals belong to.
For example in H2O, O atom belongs to Group VI (2- hence 2 lines) while
H atom is unplaced but has 1 lone electron (1- hence 1 line).

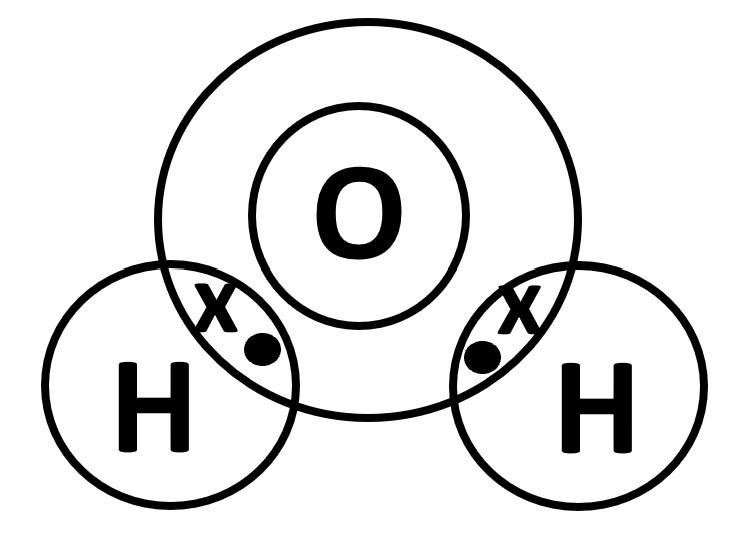
STEP 2
Identify the Period the non-metals belong to and overlap the outermost electron shells.
O atom belongs to Period 2 hence draw 2 concentric circles while
H atom belongs to Period 1 hence draw 1 circle
(Ensure that the outermost circles overlap to house the shared electrons as shown below.)
Single bond = 1 pair of shared electrons. 'x•'
Double bond = 2 pairs of shared electrons. 'x•x•'
Triple bond = 3 pairs of shared electrons. 'x•x•x•'
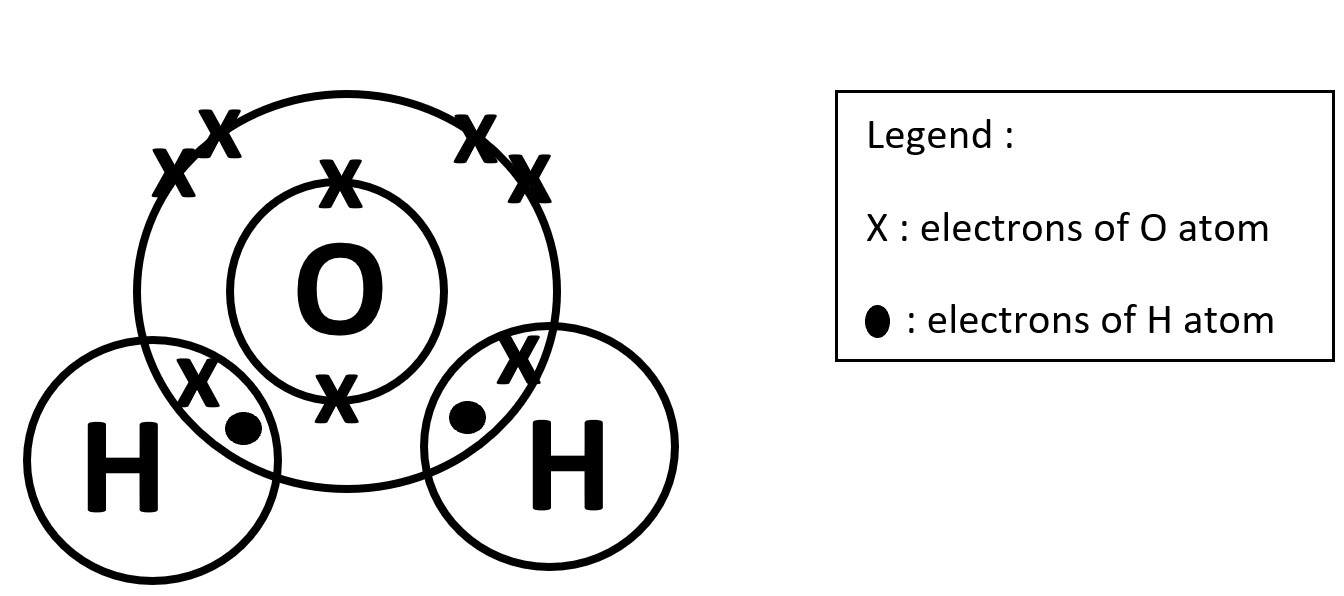
STEP 3
Fill in the Remaining Unbonded electrons for the elements and include the Legend.
In this case for O, 4 'x' electrons for outermost shell and 2 'x' electrons in the first shell.
Count the total number of outermost electrons of each atom. O atom is 8 while H atom is 2.
The stable noble gas electronic configuration of the outermost electrons is either 8 or 2.