General rule of the thumb : Metal + Non-metal --> Ionic
STEP 1
Identify the Period and Group the metal ion and non-metal ion belong to.
For example in LiCl, Li atom belongs to Period 2 (hence 2 concentric circles) while Cl atom belongs to Period 3 (hence 3 concentric circles).
But for the Li+ ion, draw 1 electron shell less meaning draw only 1 circle. For Cl- ion, draw 3 electron shells (No change in number of electron shells). Include charge and symbol as shown in the diagram below.
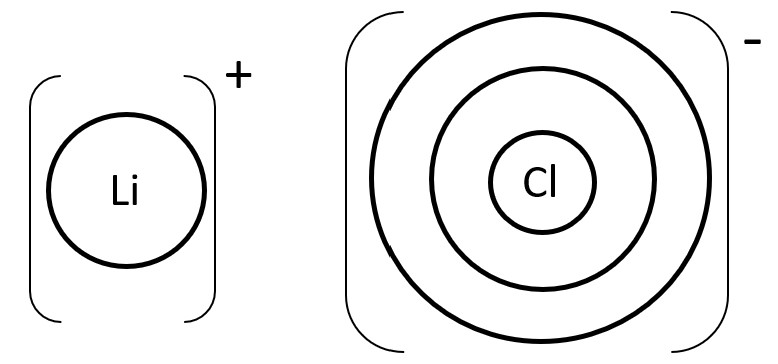
STEP 2
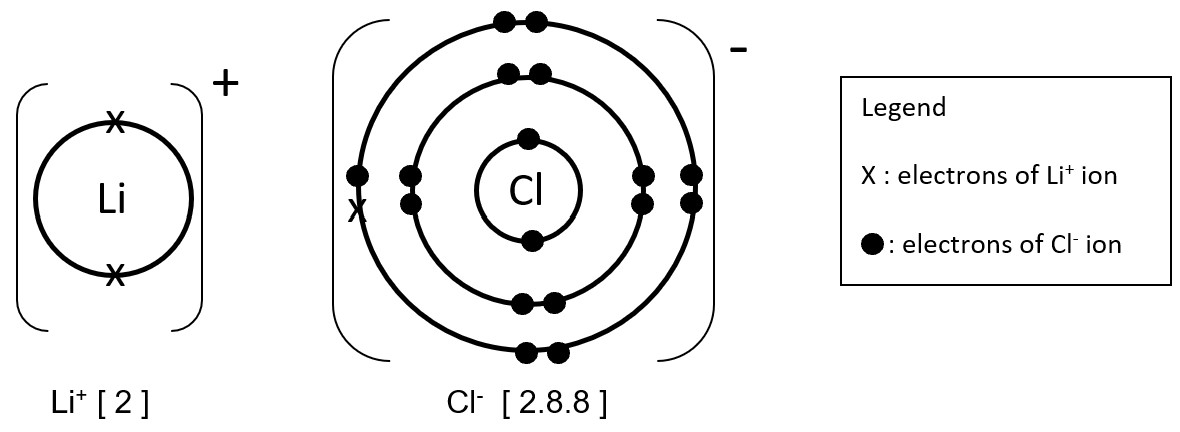
Add 'x' to Li+ ion and '•' to Cl- ion. The 'dot' and 'cross' represents electrons of the respective ions.
Note that Li (the metal) ion contains only 1 type of electron 'x' while Cl (the non-metal) ion contains 2 types of electrons 'x' and '•'.
Ensure that the outermost shell (highlighted in blue) is either 8-electron (octet) or 2-electron (duplet) state.

STEP 3
Include the Legend and write down the electronic configuration of Li+ ion and Cl- ion as shown below.