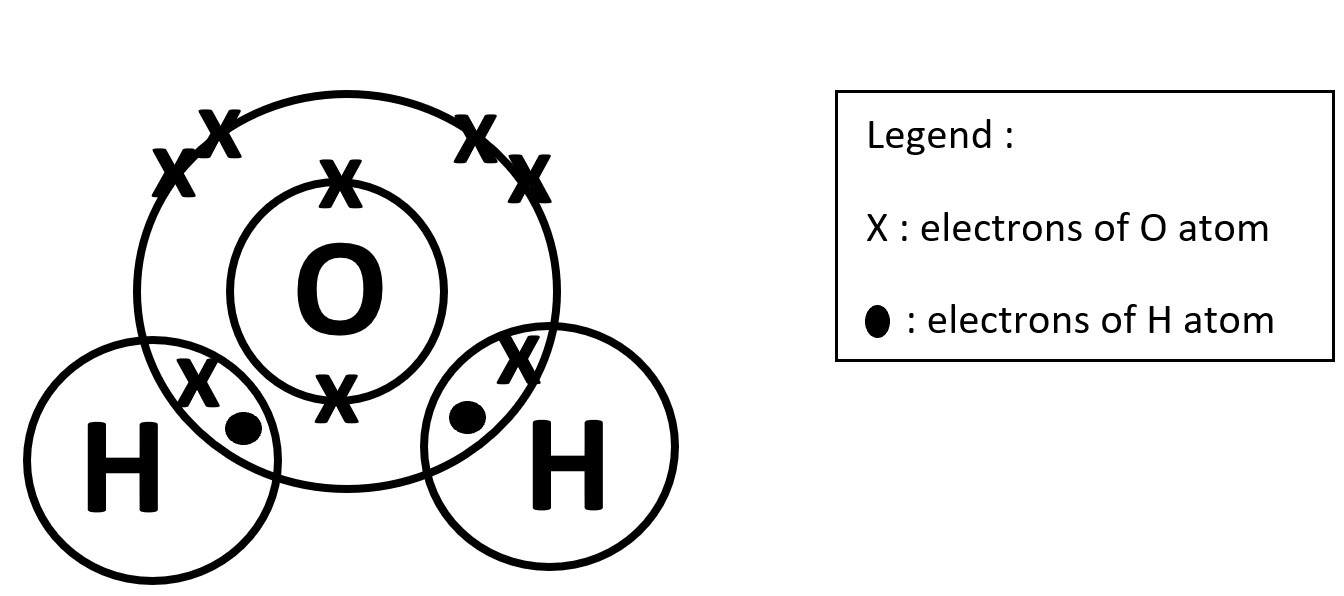
General rule of the thumb : Non-metal + Non-metal --> Covalent
STEP 1
Identify the Group the non-metals belong to.
For example in H2O, O atom belongs to Group VI (2- hence 2 lines) while
H atom is unplaced but has 1 lone electron (1- hence 1 line).

STEP 2
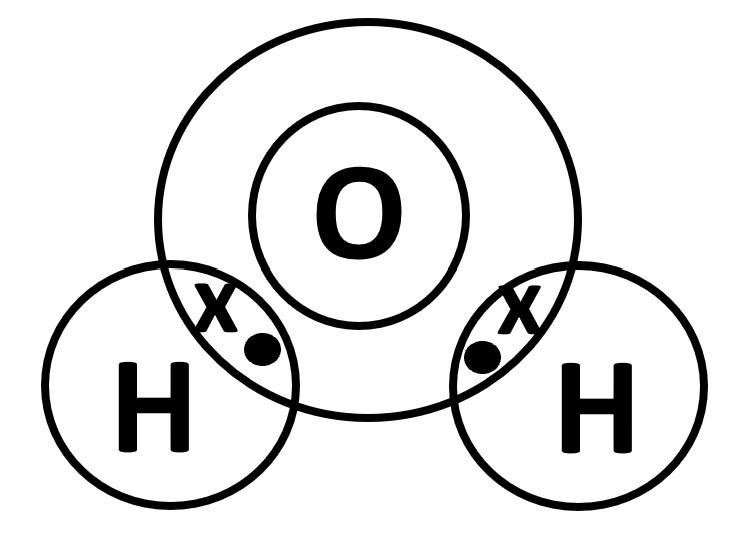
Identify the Period the non-metals belong to and overlap the outermost electron shells.
O atom belongs to Period 2 hence draw 2 concentric circles while
H atom belongs to Period 1 hence draw 1 circle
(Ensure that the outermost circles overlap to house the shared electrons as shown below.)
Single bond = 1 pair of shared electrons. 'x•'
Double bond = 2 pairs of shared electrons. 'x•x•'
Triple bond = 3 pairs of shared electrons. 'x•x•x•'
STEP 3
Fill in the Remaining Unbonded electrons for the elements and include the Legend.
In this case for O, 4 'x' electrons for outermost shell and 2 'x' electrons in the first shell.
Count the total number of outermost electrons of each atom. O atom is 8 while H atom is 2.
The stable noble gas electronic configuration of the outermost electrons is either 8 or 2.